Heap leaching is a transformative mining technique that has revolutionized the extraction of metals like copper, gold, and uranium from low-grade ores. As traditional mining methods become less economically viable for certain deposits, heap leaching provides an efficient and cost-effective alternative for recovering valuable metals from ore that would otherwise be considered waste. This process involves stacking crushed ore into large heaps and applying specialized chemical solutions that dissolve the metals, allowing them to be collected and processed.
Today, heap leaching accounts for over 30% of the world’s copper and gold production, representing a significant increase from approximately 3% several decades ago. This growth is driven by rising demand for metals and the depletion of high-grade ore sources. Typical heap leach recovery rates for copper range from 60% to 70%, while oxide minerals can achieve higher recoveries, between 75% and 95%, within 30 to 100 days.
In this blog, we will explore what heap leaching is, how it works, its significance in modern mining, and the environmental considerations associated with it. Whether you’re a mining professional, student, or curious reader, understanding heap leaching is key to appreciating the innovations driving resource recovery today.
What is Heap leaching?
Heap leaching is a metallurgical process for extracting metals from low-grade ores by stacking crushed ore, mined from deposits, into heaps and irrigating them with leaching solutions. The solution percolates through the heap, dissolving valuable metals which are collected at the base as pregnant leach solution (PLS) for further recovery.
The PLS is a concentrated solution containing dissolved metals.
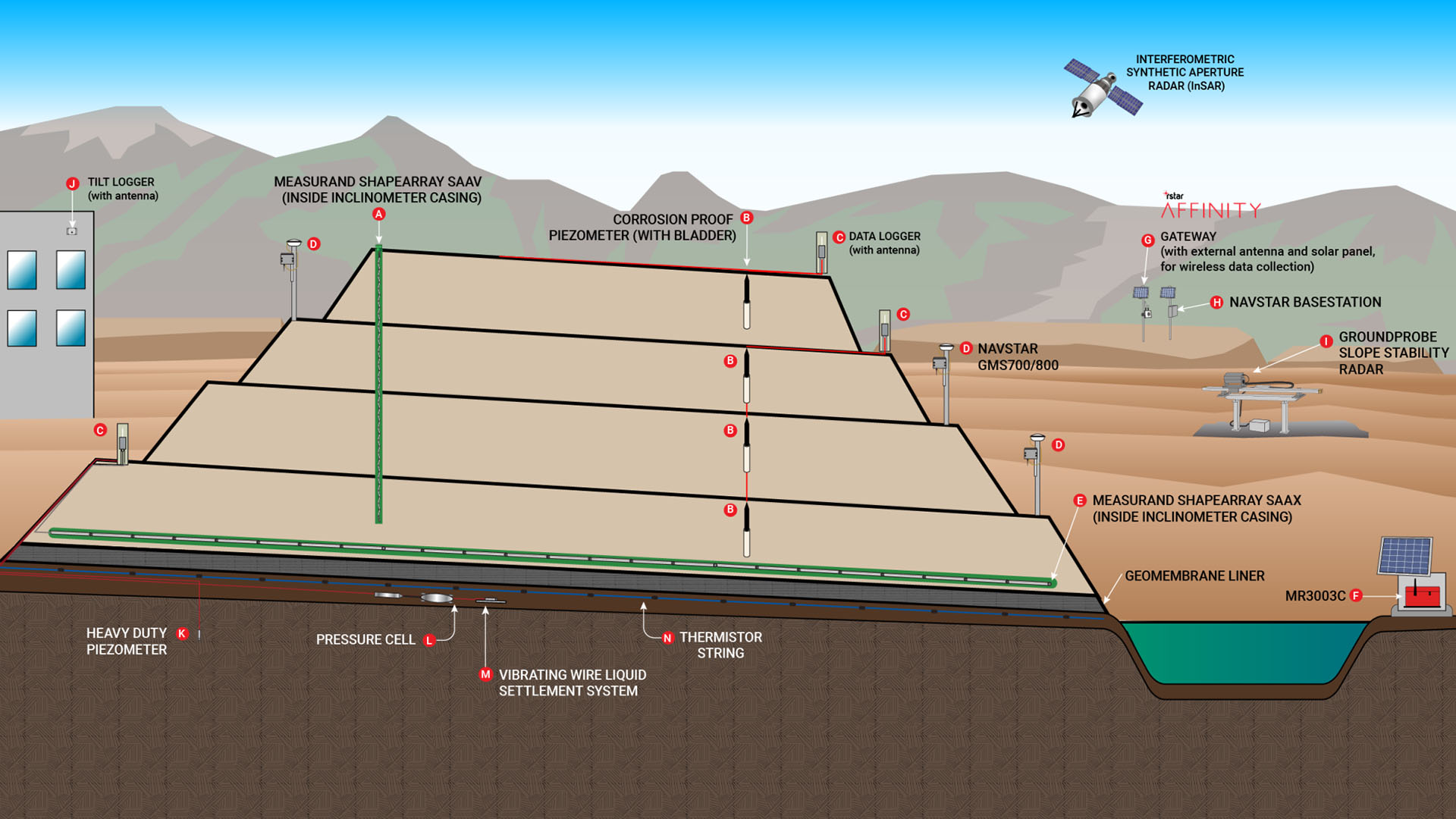
Why does heap leaching matter?
It enables economic recovery of metals from ores that are too low in grade for traditional methods. According to the U.S. Geological Survey, heap leaching accounts for over 20% of global copper production. Some operations produce hundreds of thousands of tonnes of metal annually through this mining process.
Heap leaching also plays a major role in gold extraction from oxide ores. Recovering gold from low-grade ores using heap leaching is a key application of this technique.
The History of Heap Leaching
Heap leaching was pioneered in the 1960s in the United States for copper oxide ores in several large mines. Dump leaching, a related technique, was also used historically before the development of modern heap leaching methods. Its adoption grew rapidly in gold mining in the 1970s and 1980s, particularly in Nevada. Today, heap leaching is widely used worldwide for the recovery of copper, gold, silver, and uranium, and dedicated processing plants have been established to handle the increasing volume of leach solutions.
Supporting Cluster Post to Include: [History of Mining Processes]
Introduction to Mining Techniques
Heap leaching stands out as a vital mining technique for extracting valuable metals from low grade ores that would otherwise be uneconomical to process.
The heap leaching process involves stacking crushed ore into a large heap and applying a leaching solution such as sulfuric acid for copper or cyanide for gold over the pile. As the solution trickles through the heap, it dissolves metals from the ore particles. This metal-rich liquid, known as the pregnant leach solution, is then collected at the base of the heap for further processing and metal recovery.
The heap leach process is favored in mining for its cost-effectiveness and ability to efficiently extract minerals from vast quantities of ore, making it a cornerstone method for recovering copper, uranium, and gold from low grade deposits.
Terms to Know
- Heap Pad: Engineered base with impermeable liners to prevent leakage.
- Pregnant Leach Solution (PLS): Metal-rich solution collected at the base.
- Barren Solution: Solution after metals have been recovered.
- Lixiviant: The chemical solution used (e.g., sulfuric acid for copper, cyanide for gold).
- Agglomeration: The process of binding fine ore particles together to improve heap permeability and leaching efficiency.
- Bulk Density: The mass of ore per unit volume, important for heap stability and solution flow.
- Crushing: The mechanical process of reducing ore particle size to enhance leaching.
- Percolation: The movement of leaching solution through the heap, dissolving target metals.
- Uniform Distribution: Even application of the leaching solution across the heap to maximize metal recovery.
Supporting Cluster Post to Include: Mining Glossary
Types of Ores Suitable for Heap Leaching
Heap leaching is particularly effective for processing a range of ores, especially those with low metal content. Copper ores, gold ores, and uranium ores are among the most commonly treated using the heap leaching process.
For example, copper ores are often leached with sulfuric acid, while gold ores are typically treated with a cyanide solution. This method is especially valuable for low grade ores and ore fines small particles that might otherwise be discarded as waste.
By applying heap leaching, mining operations can extract metals from these materials, increasing overall recovery and reducing the amount of waste generated. This approach not only maximizes resource utilization but also makes the extraction of metals from previously uneconomical-grade ores both practical and profitable.
The Pros and Cons of Heap Leaching
Pros:
- Low capital and operating costs.
- Suitable for low-grade ores.
- Scalable and simple design.
- Advancements in technology have enabled a more efficient leach, improving metal recovery rates.
Cons:
- Slower recovery rates than milling.
- Environmental risks (leakage, cyanide management).
- Limited to ores amenable to leaching.
- Leaching efficiency may decrease with increasing heap depth or over extended operation times.
- Greater heap depth can pose engineering and environmental challenges.
- The time required for complete metal extraction is longer than that of other methods.
Examples of Heap Leaching
- Copper (Chile, USA): Major source of cathode copper from oxide ores. In these operations, ore is often heaped in large piles on lined pads, allowing leaching solutions to percolate through the material for efficient copper extraction.
- Gold (Nevada, USA): Cyanide heap leaching revolutionized the gold mining industry. The material used for heap leaching can range from coarse rock to fine powder, depending on the type of ore, which affects how the leaching solution interacts with the ore and the overall recovery rate.
- Uranium (Kazakhstan): Acid heap leaching is widely used. The leaching process can result in the formation of soluble salts, which must be managed during solution treatment to ensure efficient uranium recovery and environmental safety.
How Heap Leaching Works
- Ore Preparation: Ore crushed to optimal size.
- Heap Construction: Ore stacked on lined pads.
- Leaching: Lixiviant sprayed over heaps to dissolve metals; the solution leaches metals from the ore as it percolates through the heap.
- Collection: PLS gathered in ponds.
- Recovery: Metals extracted by SX-EW, carbon adsorption, or precipitation; the remaining ore is often washed to recover any residual metals.
The leaching process relies on chemical reactions between the lixiviant and minerals, often involving oxygen and iron as key reactants.
Example: In gold mining, cyanide solution dissolves gold, which is later recovered using activated carbon. The leaching solution interacts with the surface of ore particles, gradually penetrating the rest of the material.
Leach Pad Design and Construction
A well-designed leach pad is essential for a successful heap leaching operation. The leach pad serves as the foundation for the ore heap and must be engineered to support the weight of the stacked ore while ensuring that the leaching solution is efficiently collected and contained.
Typically, the leach pad is constructed with a carefully prepared base, an impermeable liner—often made of synthetic materials to prevent the leaching solution from seeping into the ground, and a layer of coarse rock to promote even distribution of the solution.
The pad is built with a slight slope to facilitate drainage, allowing the percolating solution to flow toward collection points. This design ensures that the chemical reactions necessary for metal extraction occur efficiently, while also protecting the surrounding environment from potential contamination.
Environmental Concerns and Regulations
Environmental management is a critical aspect of any heap leaching operation. The use of chemicals such as cyanide and sulfuric acid in the heap leaching process can pose significant risks if not properly controlled.
Waste products, including barren solution and ore fines, must be handled and disposed of responsibly to prevent environmental harm. Regulatory agencies, such as the Environmental Protection Agency (EPA), have established strict guidelines for the containment, treatment, and disposal of chemicals and waste generated by heap leaching.
Mining operations are required to implement measures such as lined leach pads, solution collection systems, and water treatment facilities to minimize the risk of leaks and contamination. By adhering to these regulations and employing best practices, heap leaching operations can reduce their environmental footprint while maintaining efficient metal recovery.
Tips and Reminders for Heap Leaching
- Maintain solution flow uniformity to maximize recovery.
- Monitor heap stability and liner integrity.
- Manage and detoxify spent solutions and effluent before discharge. Properly manage effluent to prevent environmental contamination and ensure compliance with relevant regulations.
- Select stable, compatible soil for leach pad foundations to minimize the risk of liner failure.
Analyzing Heap Leaching
Metrics include:
- Recovery Rate: Percentage of metal recovered.
- Leach Cycle Time: Duration required for efficient extraction.
- Solution Management: Balance between PLS production and barren solution recycling.
- Percolation Rate: The Speed at which leaching solutions percolate through the heap, affecting extraction efficiency.
Percolation is a key factor in heap leaching, as it determines how effectively leaching solutions percolate through the ore, impacting the overall recovery of metals. Increased heat within the heap can accelerate chemical reactions and improve metal recovery.
Stat: Gold heap leaching operations typically achieve 60–80% recovery rates, while copper operations can exceed 85% recovery.
Supporting Cluster Post to Include: [Mining Economics]
Resources for Heap Leaching
- Books: Gold Heap Leach Operations by Daniel W. Kappes.
- Organizations: U.S. Geological Survey (USGS), SME.
- Reports: World Gold Council heap leaching studies.
Closing
Heap leaching has revolutionized metal extraction by providing a low-cost, scalable, and efficient method for recovering copper, gold, and other metals from low-grade ores. Its role in modern mining is indispensable.
Call-to-Action: Explore our [Guide to Leaching Methods] for insights into heap, in-situ, and agitation leaching.






